We Go The Extra Distance For Our Clients
Class Action and Product Liability Practice Areas
Sandy Springs Hip Replacement Lawsuit Attorney
The Sandy Springs hip replacement lawsuit attorneys at Ashenden & Associates are experienced in handling hip implant cases involving defective hip and joint replacements. Total hip replacement surgery is intended to improve mobility and relieve pain. Still, when implants fail due to design flaws, manufacturing defects, or other issues, it can lead to severe complications such as implant loosening, dislocation, or metal poisoning. Our defective hip replacement lawyers understand the complexities of hip replacement and multidistrict litigation and are dedicated to advocating for victims of defective hip devices and other joint replacement devices.
If you’ve experienced hip replacement complications from an FDA medical device or hip replacement procedure, the Sandy Springs personal injury attorneys at Ashenden & Associates can help you pursue financial compensation for medical expenses, lost wages, pain and suffering, and other damages. Our hip replacement lawsuit attorneys are committed to holding faulty medical device manufacturers accountable and ensuring you receive the justice and support you deserve.
Call our Sandy Springs hip replacement lawsuit attorneys at 770-394-8909 to schedule a free case review.
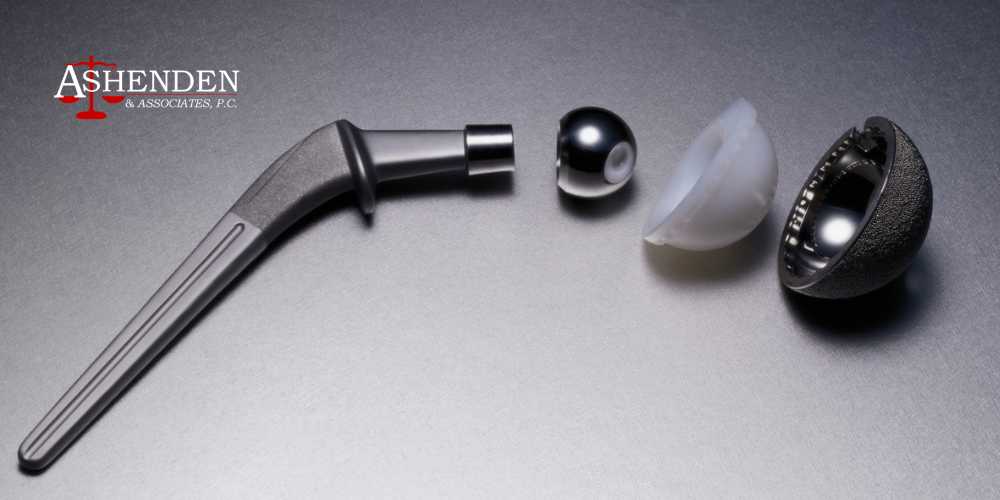
Hip Replacement Recalls
Several hip devices have been recalled in recent years due to serious complications. Some of the most prevalent hip replacement recalls from the Food and Drug Administration include:
DePuy ASR Hip Resurfacing System
Smith & Nephew Birmingham Hip Resurfacing Systems
Stryker Rejuvenate and ABG II Modular Neck Systems
Wright Medical Technology Conserve Plus and Profemur Z Hip Stem
Zimmer Durom Acetabular Cup
Individuals with metal-on-metal hips should research their implant model and manufacturer to ensure their metal hip implants haven’t been recalled. If you’ve had a metal hip replacement and your metal hip implants are on the recall list, contact your doctor immediately. They can help evaluate the hip devices and understand how the recall affects you.
Hip Replacement Lawsuit Updates 2024
There are many hip replacement manufacturers that users are suing due to their defective products. As of March 2024, there are hip lawsuits pending against the following hip replacement companies:
Exactech
DePuy Synthes
Smith & Nephew
Stryker
Synovo
Zimmer
If you have received a hip replacement implant from any of these companies or experienced any hip implant complications, speak with an attorney regarding your case. They can help provide you with updated information on the current status of any ongoing legal action.
What Are Hip Implants?
Hip implants, hip prostheses, or hip replacements are medical devices used to replace a damaged or diseased hip joint with artificial components. The hip joint is a ball-and-socket joint that connects the thigh bone (femur) to the pelvis, which allows the leg to move in multiple directions.
Like other joints, the hip joint contains cartilage, a connective tissue that lines the surfaces of the bones, helping reduce friction and cushion the joint during motion. When this cartilage wears down or the bones near the hip joint become damaged or infected, hip movement can be painful, difficult, and even impossible.
Hip replacement surgery allows doctors to replace the hip joint with a metal implant, which aims to alleviate pain, improve mobility, and restore function to the joint. This kind of procedure is usually recommended for individuals with conditions such as osteoarthritis, rheumatoid arthritis, or hip fractures.
Parts of a Hip Replacement Device
Almost every hip replacement device consists of the same components, including the:
Acetabular socket, which is located on the side of the pelvis
Femoral head, which is a ball-shaped device made to mimic the shape of the top of the femur
Femoral stem or neck stem, which is inserted into the femur and connects the femoral head to the femur
Many hip implants use metal components due to their potential longevity. Still, many individuals with metal-on-metal hips have experienced complications and even premature implant failure despite their predicted longevity.
Some hip replacement implants may include a polyethylene liner between the socket and femoral head, which can help reduce the risk of metal-on-metal complications. However, some of these liners have also been linked to complications, including premature implant failure.
Hip Replacement Manufacturers With Pending Hip Implant Lawsuits
There are many different medical device manufacturers that are currently being sued as a result of their hip replacement implants. If you or a loved one has a hip replacement device from any of these companies, contact a hip replacement lawyer near you who can help you understand your legal rights.
Exactech Hip Replacement Lawsuits
Unlike some metal hip replacement manufacturers, Exactech designed their implants to have a plastic liner between the femoral head (the top of the thigh bone) and an acetabular cup (the socket in the hip). These GXL liners were used in Novation, Acumatch, and MCS hip replacement devices.
While these liners were initially designed to make the implants last longer, these GXL liners have been linked to premature deterioration and implant failure. Many individuals with Exactech hip replacement devices have had to undergo revision surgery, which can be painful, expensive, and emotionally taxing.
DePuy ASR Hip Replacement Lawsuits
Another defective hip replacement manufacturer that faced legal action is DePuy Synthes, a medical device company under Johnson & Johnson with multiple hip replacement devices. While the company already agreed to a $1 billion settlement over their defective Pinnacle hip implants, DePuy still faces legal trouble due to their ASR hip resurfacing system.
The metal-on-metal DePuy hip replacements have been found to release small metal particles into the soft tissue surrounding the hip joint, causing these tissues to die. The embedded particles could also move into the patient’s bloodstream, leading to metallosis, also known as metal poisoning.
Contact our Sandy Springs DePuy hip replacement lawsuit attorney team for a free consultation.
Smith & Nephew Hip Replacement Lawsuits
Many individuals with the Smith & Nephew Birmingham hip resurfacing implants have filed lawsuits against the Smith & Nephew for complications they suffered as a result of the implants. Their metal-on-metal hip resurfacing devices were linked to excessive and premature wear, as well as metallosis, which can lead to a wide range of health complications. This led Smith & Nephew to recall these devices in 2015.
Stryker Hip Replacement Lawsuits
Multiple Stryker hip implant devices have been recalled due to adverse health complications: the LFIT Anatomic CoCR V40 femoral head devices and the Rejuvenate and ABG II hip implant devices.
Stryker LFIT Anatomic CoCR V40 Hip Implants Lawsuit
Patients have experienced problems with Stryker’s LFIT Anatomic CoCR V40 femoral head devices, which are placed at the end of the implant’s neck stem. This femoral head has been found to corrode prematurely, which can cause the head to dislocate from the neck stem. Additionally, this corrosion has led to problems with metal poisoning, which can cause other health issues.
Stryker Rejuvenate and ABG II Hip Implants Lawsuit
The Stryker Rejuvenate modular-neck stem was designed to provide better implant stability and make the installation process easier for doctors. The same is true for the Stryker ABG II modular-neck hip stems.
Unfortunately, these medical devices are also prone to corrosion, which can cause small metal particles to be embedded in the tissue surrounding the hip. This corrosion has been found near the modular-neck junction. The modular-neck junction allows doctors to change the length of the hip replacement implant to suit their patient’s individual physiology better.
Synovo Hip Replacement Lawsuits
The U.S. Food and Drug Administration (FDA) has warned consumers and medical providers about the potential risks of Synovo hip resurfacing systems. The Food and Drug Administration claims that Synovo changed the design of its acetabular bearings, acetabular fixation cups, and femoral resurfacing cups without proper FDA approval.
If you have a Synovo hip replacement device implanted after 2019, it’s possible that your hip implant is not approved by the Food and Drug Administration, which could lead to potential health issues. If you have not experienced any complications, it’s essential to have your physician or orthopedic surgeon closely monitor your implant for corrosion, failure, or other complications.
If you have experienced any complications from your Synovo hip replacement, contact an attorney as soon as possible. They can help you understand your legal options in this situation.
Wright Medical Group Hip Replacement Lawsuits
Like other metal-on-metal hip implants, the Wright Medical hip implant devices have been associated with corrosion and implant failure. Users have reported experiencing issues with the company’s Conserve, Dynasty, Lineage, and Profemur hip implant devices. To settle hip replacement MDL claims made against them, the Wright Medical Group agreed to two massive settlements in 2016 and 2017, which cost the company more than $300 million in total.
Zimmer Hip Replacement Lawsuits
Patients have also reported experiencing complications with Zimmer hip replacement products, including their Durom Cup, M/L Taper Hip Prosthesis, and VerSys Femoral Head. Many of these lawsuits are in relation to other complications associated with metal-on-metal hip replacements, like metallosis and premature failure. While many of the Durom Cup-related claims have been handled, the legal disputes over the M/L Taper Hip Prosthesis, and VerSys Femoral Head are ongoing.
Why People Are Filing Hip Replacement Lawsuits
People are filing metal-on-metal hip replacement lawsuits due to concerns over potential complications and adverse effects associated with these devices. These include issues such as metallosis (metal poisoning), tissue damage, implant failure, and the need for revision surgery.
Before bringing a medical device to market, manufacturers must conduct rigorous testing and obtain regulatory approval to demonstrate that their product is safe and effective for its intended use. They are also responsible for providing adequate warnings and instructions for patients and healthcare providers.
Manufacturers may be liable for the resulting harm when complications arise from defective hip implants, such as design flaws, manufacturing defects, or inadequate warnings about potential risks.
Current allegations against the above manufacturers suggest they failed to warn patients and physicians about these risks adequately. This has led users and medical professionals to take legal action to seek compensation for medical expenses, pain and suffering, and other damages.
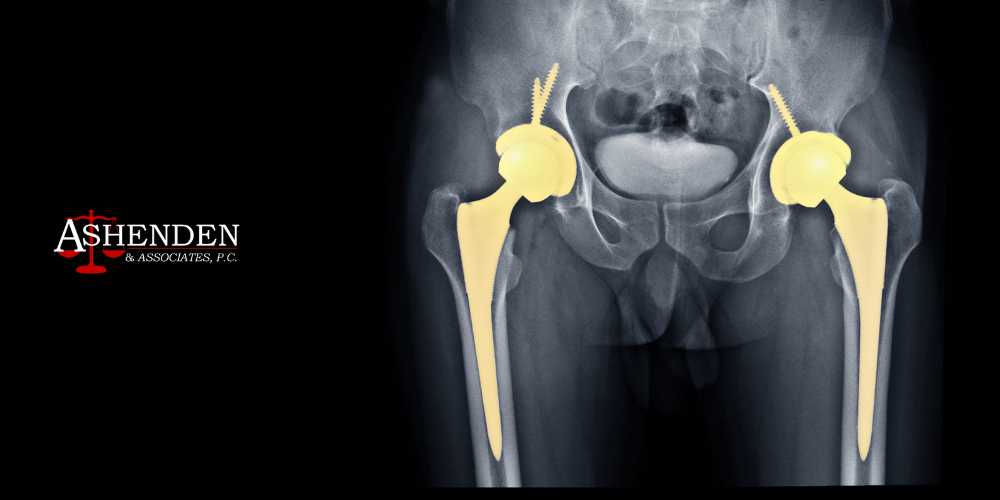
Hip Replacement Complications
One of the most dangerous and most common complications associated with metal-on-metal hip replacements is metallosis or metal poisoning. When the leg moves, the metal femoral head grinds against the metal acetabular socket, which can cause tiny metal particles to shed from the implant. This can cause the metal to become embedded in the soft tissues surrounding the hip joint or cause the metal to enter the bloodstream. This is metallosis.
Metallosis is associated with several serious health complications, including localized symptoms around the hip joint and issues with the heart, skin, nervous system, and thyroid. Symptoms of metallosis from faulty hip implant devices include joint instability, audible clicking sounds from the hip, and new joint pain that wasn’t present after surgery.
Additionally, many users have filed hip replacement lawsuits over premature implant failure, which occurs when the implant gives out long before it’s predicted to. This can require patients to undergo revision surgery to have the implant replaced or repaired, which can be costly, time-consuming, and physically demanding.
Signs of Hip Implant Failure
Hip implant failure occurs when the implant fails to function properly, causing pain, discomfort, or decreased mobility in the hip joint. Signs of hip implant failure include:
Persistent or increasing pain in the hip, groin, or thigh area
Swelling, inflammation, or tenderness around the hip joint
Difficulty or discomfort when walking, standing, or bearing weight on the affected hip
Instability or a sensation of the hip giving way
Audible clicking, popping, or grinding noises coming from the hip joint
Limited range of motion or stiffness in the hip joint
Visible changes such as deformity, asymmetry, or swelling around the hip
Radiating pain or discomfort extending to the buttocks, lower back, or down the leg
Signs of infection, such as fever, warmth, redness, or drainage at the surgical site
Signs of Hip Implant Rejection
Like all medical implants, hip replacements can be rejected by the body, triggering the body’s immune response to protect the body from foreign materials. Signs of hip implant rejection may include:
Persistent or worsening pain in the hip or groin area
Swelling, redness, warmth, or tenderness around the hip joint
Inflammation or fluid buildup at the surgical site
Difficulty or discomfort with movement, such as walking or bending
Instability or a feeling of the hip joint being loose
Limited range of motion in the hip joint
Fatigue or general malaise
Systemic symptoms such as fever or chills
Delayed wound healing or infection at the surgical site
Longterm Effects of Defective Implants
While hip replacements are often considered safe, healthy ways to restore hip function, several long-term complications are associated with certain defective implants. The long-term effects of defective hip implants can be severe and may include chronic pain, decreased mobility, and impaired quality of life.
Complications such as metallosis, tissue damage, and implant failure can lead to the need for multiple revision surgeries, which carry their own risks and complications. Additionally, patients may experience emotional distress and financial burdens as a result of any ongoing medical monitoring to manage their defective implant.
How to Know if You’re Eligible for a Hip Replacement Lawsuit in Atlanta or Sandy Springs, GA
You may be eligible for a hip replacement lawsuit if you have had a recalled hip replacement implant and have experienced health complications as a result of your hip implant. A product liability lawyer can help evaluate your eligibility and determine whether or not you have grounds to file a hip replacement lawsuit.
If you live in Sandy Springs or the greater Atlanta area, the product liability lawyers at Ashenden & Associates are here to help ensure you receive the compensation you deserve. Our dedicated lawyers will review your medical records and other relevant documentation to help determine whether or not you’re eligible to file a lawsuit against the manufacturer of your hip implant.
How to File a Hip Replacement Lawsuit for Faulty Hip Implants
Contact an attorney regarding your case if you’re looking to file a hip replacement lawsuit over a faulty hip implant. They can help gather the necessary evidence, negotiate to help secure a settlement, and even represent you in court if needed.
Many product liability lawsuits are handled in multidistrict litigation (MDL) settings, consolidating hip implant cases from multiple victims over the same product and similar complications. Depending on the status of any ongoing litigation, an attorney can also help you join multidistrict litigation against negligent hip replacement manufacturers, ensuring you receive compensation from global hip replacement settlements if one is reached.
Georgia Statute of Limitations for Hip Implant Lawsuits
Like with other product liability claims, the statute of limitations for filing hip replacement lawsuits regarding these joint replacement devices is two years from the date of injury or the date the injury was discovered. Those who haven’t filed hip replacement lawsuits within this timeframe may lose their right to seek compensation for their losses.
Compensation for Victims of Recalled Hip Replacement Devices
Victims of defective hip systems or devices may be able to recover various types of compensation in hip replacement settlements. This can include reimbursement for losses such as:
Medical expenses
Lost wages
Loss of quality of life
Pain and suffering
Emotional distress
Rehabilitative costs
Assistive technology
The amount of compensation a victim can recover in a hip replacement lawsuit depends on multiple factors, including the extent of serious complications experienced, their losses, and the skill of their legal representation.
If you or a loved one have experienced complications as a result of your hip implant, contact the hip replacement lawsuit attorneys at Ashenden & Associates. Our attorneys have a long history of successful case results, so you can rest assured that your case will be in capable hands. Call today for a free case review.
Sandy Springs and Atlanta Hip Implant Lawsuit Attorneys
Filing hip replacement lawsuits against a major hip replacement manufacturers can be difficult, which is why those with defective bags, defective hip implant parts, or defective hip implants should turn to a trusted Sandy Springs hip replacement lawsuit attorney who can help them with their case. At Ashenden & Associates, P.C., our hip replacement lawyers are dedicated to helping victims of defective hip devices hold hip replacement manufacturers accountable and recover compensation for their losses.
If you or a loved one has suffered due to a defective hip implant, contact our Sandy Springs hip replacement lawyer team regarding your case. We provide personalized attention and compassionate support to everyone who walks through our doors, guiding them through the legal process with professionalism and integrity.
Let us be your personal legal advocates–call us at 770-394-8909 or contact us via our website to schedule a free case review with a member of our team.
