We Go The Extra Distance For Our Clients
Class Action and Product Liability Practice Areas
Sandy Springs Hernia Mesh Lawsuit
The Sandy Springs hernia mesh lawyers at Ashenden & Associates are dedicated to representing hernia mesh victims who have suffered complications from defective hernia mesh products. Defective medical devices can lead to severe pain, infection, or other serious complications requiring revision surgery or long-term medical care. Our Sandy Springs hernia mesh lawsuit lawyers are committed to helping victims navigate the complexities of defective hernia mesh claims, pursuing compensation for medical bills, lost wages, and pain and suffering.
Our Georgia hernia mesh lawyer team understands the challenges faced by our clients and works tirelessly to hold hernia mesh manufacturers accountable for unsafe medical products. If you’ve experienced serious complications from hernia mesh surgery, the hernia mesh lawsuit attorneys, Ashenden & Associates, are here to provide you with the experienced legal representation you deserve.
Our Sandy Springs personal injury attorneys at Ashenden & Associates have decades of collective experience litigating claims for defective medical devices. Our Sandy Springs hernia mesh lawsuit lawyers are dedicated to helping our clients navigate these challenging times and secure the justice and financial restitution they deserve.
If you believe you’ve been adversely affected by hernia mesh, you may be a candidate for a valid hernia mesh lawsuit or hernia mesh class action lawsuit. Call Ashenden & Associates at 770-394-8909 today to discuss your case and explore your legal options.
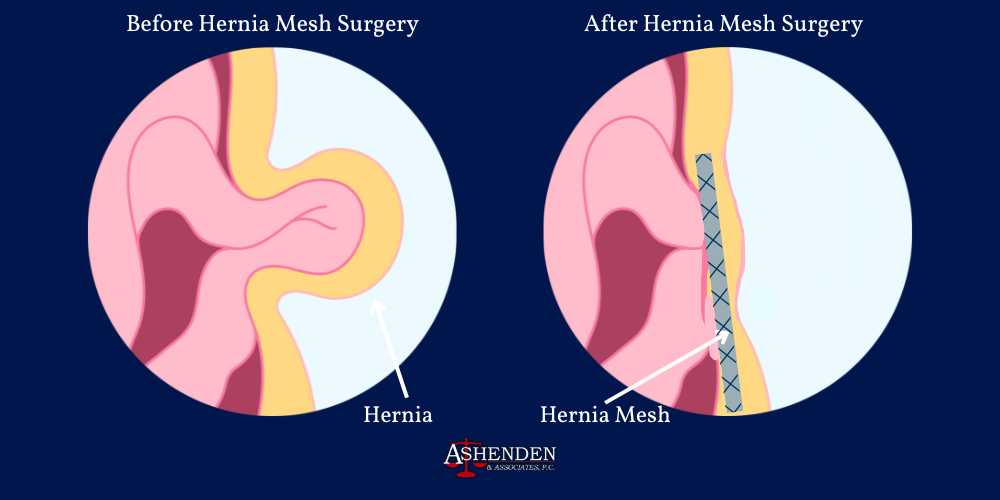
What is Hernia Mesh?
Hernia mesh is a medical device designed to provide support and reinforcement for weakened or damaged tissue during hernia repair procedures. A hernia occurs when an organ or tissue bulges through a weak spot, often in the abdominal wall.
Hernia mesh products are typically made from synthetic materials like polypropylene or other absorbable substances. The hernia mesh implants are then surgically implanted during hernia repair surgery to prevent hernia recurrence. The surgical mesh acts as a scaffold, allowing new tissue to grow through it, thereby strengthening the area and reducing the likelihood of future hernias. While hernia mesh products can be effective, some patients experience complications with the medical devices, such as infection, migration, or adhesion, necessitating additional medical intervention.
Despite its widespread use of hernia mesh products, the implementation of mesh during hernia surgery has been linked to a range of hernia mesh complications, leading to increased scrutiny and new hernia mesh lawsuits.
If you’ve experienced significant health issues and financial burdens due to complications from hernia mesh surgery, you may be entitled to file a hernia mesh lawsuit and seek compensation for your suffering. Our Sandy Springs hernia mesh lawsuit attorneys have extensive experience with Bard hernia mesh and Covidien hernia mesh cases.
Benefits of Hernia Mesh Surgery
Hernia mesh surgery offers several key benefits:
- Reduced Recurrence Rates: One of the primary advantages of using surgical mesh is its effectiveness in significantly lowering the chances of hernia recurrence. The mesh implant acts as a support structure, reinforcing the hernia mesh injury and decreasing the likelihood of hernia recurrence.
- Faster Recovery: Patients who undergo surgery for severe injuries with surgical mesh often experience shorter recovery periods. The added support of the mesh implant allows for quicker tissue healing and less postoperative pain.
- Enhanced Durability: The durability of elective incisional hernia repair surgery is greatly improved with the use of surgical mesh, providing a long-lasting solution. This is particularly beneficial for large or complex inguinal hernias, where sutures alone may not provide sufficient strength.
- Versatility: Hernia mesh products are is available in various types and designs, making it a versatile option.
- Minimal Invasiveness: Many hernia mesh surgeries can be performed laparoscopically, involving smaller incisions than traditional open surgery.
These benefits contribute to the widespread use of hernia mesh products in hernia surgery, providing a reliable method that enhances patient outcomes.
FDA Hernia Mesh Recall List
Over the years, several hernia mesh recalls have been due to serious complications that compromised patient safety. Some notable hernia mesh recalls include:
Bard Composix Kugel Hernia Patch
In 2005, Bard initiated the first of several recalls for its Kugel Patch series due to a critical flaw. This Bard hernia mesh recall was prompted by issues with the patch’s memory recoil ring, which could potentially fracture, leading to serious complications such as bowel obstruction, perforation, and fistulas.
The severity of these hernia mesh complications led the FDA to classify the recall as a Class I, representing the highest level of risk. Subsequent recalls for different Kugel Patch variants were issued between December 22, 2005, and January 10, 2007, ultimately affecting over 137,000 units. This series of recalls has fueled a wave of Bard hernia mesh lawsuits and is a key focus in the Bard hernia mesh MDL (Bard hernia mesh multi district litigation), as affected patients seek compensation for injuries caused by defective products.
Atrium C-QUR Mesh
In 2013, Atrium initiated a recall of over 145,000 units of its C-QUR hernia mesh, encompassing several models such as the C-QUR V-Patch, TacShield, Edge, and the standard C-QUR Meshes. This recall was designated as a Class 2 by the FDA, indicating a moderate health risk. The reason provided by Atrium for the recall was that elevated humidity levels could lead the mesh to adhere to the packaging’s inner liner, potentially compromising its usability and safety.
Ethicon Proceed and Physiomesh
Between 2005 and 2014, Ethicon recalled over 18,000 units of its Proceed hernia mesh due to defects that could cause “delamination,” where the mesh’s coating might peel, potentially leading to adhesions and bowel fistulas.
The FDA classified this as a Class 2 recall, indicating moderate health risk. In 2016, Ethicon withdrew its Physiomesh Flexible Composite mesh from markets in Australia and Europe after studies in Germany and Denmark showed higher rates of recurrence and reoperation compared to similar products. Instead of a formal recall in the U.S., Ethicon opted for a market withdrawal.
Gentrix Surgical Matrix
In February 2019, ACell initiated a limited recall affecting two specific sizes of its Gentrix Surgical Matrix Thick devices, specifically the 20 x 30cm and 30 x 40cm variants. The recall was prompted by the discovery that these two products did not align with the company’s established specifications for tensile strength, leading to concerns about their performance and reliability in surgical applications.
The recall was narrowly focused, involving only these two products within the Gentrix Surgical Matrix Thick line.
Parietex Composite Parastomal Mesh
In October 2018, Covidien LLC, a Medtronic subsidiary, announced a recall of 7,333 units of its Parietex Composite Parastomal Mesh. The recall was initiated after the company identified issues with surgical mesh failure occurring several years post-parastomal hernia repair, particularly when using the modified Sugarbaker technique.
Post-parastomal hernia repair surgeries are used to fix a hernia that develops around a stoma, which is an opening created during an ostomy surgery. This type of hernia repair surgery is particularly challenging because of the location and the potential serious complications involved. One of the major concerns with this is hernia recurrence, which can be more common in hernia mesh cases than in other types of hernia repairs, such as inguinal hernia repair. Patients who experience complications or hernia mesh injury during the initial repair may require revision surgery to address the issues, which can lead to further complications. While inguinal hernias are more common, post-parastomal hernias require specialized surgical approaches to minimize the risk of recurrence and ensure successful hernia mesh surgery outcomes.
This hernia mesh failure was linked to various complications, including hernia recurrence, discomfort, bulging, and alterations in the skin overlying the repair site, prompting the company to take corrective action to address these concerns.
What is the Hernia Mesh Lawsuit in Sandy Springs and Atlanta, GA?
The hernia mesh class action lawsuit encompasses legal actions taken by individuals who have suffered adverse effects following hernia repair surgeries involving mesh implants. These hernia mesh lawsuits are filed against hernia mesh manufacturers on the grounds that the medical devices have caused significant health complications, including infections, chronic pain, mesh migration, and hernia recurrence, among others.
The basis of these legal challenges often revolves around several key allegations against hernia mesh manufacturers: defective product design, defective product manufacturing, improper product labeling.
Patients can also bring a hernia mesh lawsuit against negligent physicians if the patient suffered hernia mesh complications due to medical negligence instead of defective medical devices.
Defective Product Design
Defective product design allegations in hernia mesh class action lawsuits focus on the fundamental flaws inherent in the design of the surgical mesh products themselves. Plaintiff’s hernia mesh lawyers argue that these design defects predisposed hernia mesh failure once the hernia mesh was implanted, leading to severe complications such as organ perforation, chronic pain, and the need for revision surgery.
Hernia mesh lawsuits contend that the hernia mesh manufactured was not adequately tested or designed to ensure its safety and effectiveness over time, thereby putting patients at unnecessary risk. Such hernia mesh cases aim to hold negligent manufacturers accountable for not only the physical and emotional distress caused by these design flaws but also the financial burdens incurred by affected patients due to medical bills and lost wages.
Defective Product Manufacturing
Defective product manufacturing claims in hernia mesh lawsuits address issues that arise during the production process of the mesh devices. These hernia mesh lawsuits claim that, regardless of the design’s intentions, errors or oversights in the manufacturing stage led to defects in the hernia mesh, compromising its integrity and safety. Such defects may include inconsistencies in the material composition, contamination, or deviations from the intended design specifications.
As a result, patients who received these defectively manufactured meshes may experience adverse outcomes, including infection, inflammation, and the failure of the hernia repair, necessitating additional medical interventions. These legal actions seek to hold manufacturers responsible for the oversight in quality control and the resultant impact on patients’ health and well-being.
Improper Product Labeling
Improper product labeling allegations in hernia mesh lawsuits focus on the failure of manufacturers to provide adequate warnings, instructions, or information regarding the potential risks and side effects associated with the use of their hernia mesh products. These hernia mesh lawsuits claim that patients and healthcare providers were not sufficiently informed about the possible complications, such as the risk of migration, consistent pain, or the need for revision surgery, that could arise from hernia mesh implants.
As a result, individuals affected by these oversights may have been deprived of the opportunity to make fully informed decisions regarding their hernia repair options. Legal actions based on improper product labeling seek to hold manufacturers accountable for their lack of transparency and the subsequent harm suffered by patients due to inadequate information.
Medical Malpractice
Medical malpractice claims within the context of hernia mesh lawsuits arise when healthcare professionals fail to adhere to the accepted standards of medical care during the implantation of hernia mesh devices. These claims can involve instances where the mesh was improperly implanted, the wrong type of mesh was used for a specific hernia type, or there was a failure to recognize and appropriately address complications arising post-surgery.
Patients pursuing medical malpractice claims in hernia mesh cases seek to demonstrate that their adverse outcomes were a direct result of the healthcare provider’s negligence or deviation from standard practices, leading to additional pain, suffering, and financial burden due to further medical treatments or surgeries.
If you believe you have grounds to file a medical negligence lawsuit against the medical professional who did your hernia repair surgery, contact Sandy Springs medical malpractice attorneys today.
Hernia Mesh Complications our Sandy Springs Surgical Mesh Lawyers Handle
Hernia mesh injuries and complications can vary widely in severity and may include:
Hernia Mesh Adhesion
Mesh adhesion occurs when a hernia mesh implant abnormally attaches to internal organs or tissues, forming scar-like tissue that binds these surfaces together. This complication can cause significant discomfort, restricted mobility, and impair the function of the affected organs.
Patients with mesh adhesions may experience chronic pain, digestive problems, and in severe cases, life-threatening conditions requiring emergency surgery. Adhesions post hernia repair not only reduce the patient’s quality of life but also often require additional medical treatment, leading to increased physical and financial burdens.
Bowel Obstruction
Bowel obstruction is a serious complication that can occur when hernia mesh causes a blockage in the intestines. This can happen if the mesh migrates, forms adhesions with the bowel, or induces scar tissue that constricts the intestinal passage. Patients experiencing bowel obstruction may suffer from severe abdominal pain, vomiting, constipation, and an inability to pass gas, indicating a potentially life-threatening condition that requires immediate medical attention.
Hernia Recurrence
Hernia recurrence occurs when a hernia reappears at the same site after surgical repair, often indicating that the initial procedure, possibly involving the hernia surgery mesh, did not provide a permanent solution. This can result from several factors, including the failure of the mesh to properly integrate with the tissue, mesh migration, or inadequate surgical technique
For patients, a recurrent hernia often means experiencing the original symptoms again, such as pain and discomfort, along with the psychological distress of facing another surgery.
Hernia Mesh Infection
Infection is a common risk associated with any surgical procedure, but hernia mesh implants can sometimes exacerbate this risk. Mesh infections can occur when bacteria colonize the implant, either during surgery or from post-operative complications. These infections can be particularly challenging to treat due to the presence of the foreign material, which may harbor bacteria and shield them from antibiotics.
For patients, an infection related to hernia mesh can lead to severe pain, swelling, redness, and fever. In some cases, the infection can spread, leading to more systemic symptoms and requiring aggressive antibiotic therapy or even surgical removal of the mesh to fully eradicate the infection.
Hernia Mesh Failure
Hernia mesh failure in the context of hernia repair refers to the breakdown or malfunctioning of the hernia mesh implant post-surgery. This can manifest as the mesh tearing, fraying, or losing its structural integrity, which compromises its ability to support the repaired hernia site effectively. Such failures can result from the body’s reaction to the mesh material, manufacturing defects, or the mechanical stresses placed on the mesh over time.
For patients, hernia mesh failure can lead to a recurrence of the hernia, pain, and the potential for other complications such as adhesions or perforations. Hernia mesh failure often necessitates additional surgical interventions to remove or replace the faulty mesh, leading to further physical discomfort, prolonged recovery periods, and increased medical expenses, significantly impacting the patient’s quality of life and financial stability.
Mesh Migration
Mesh migration is a complication where a hernia mesh implant shifts from its original position to another part of the body. This can happen due to improper mesh fixation during surgery, natural body movements, or gradual mesh detachment. Mesh migration can cause tissue irritation, fistulas (abnormal connections between organs), and even organ damage if the mesh erodes into surrounding structures.
Chronic Pain
Chronic pain following hernia mesh surgery is a persistent discomfort that can last for months or even years after the procedure. This pain can arise from various factors, including nerve damage or entrapment, inflammation caused by the body’s reaction to the hernia surgical mesh implants, or complications related to the mesh implant itself, such as migration or adhesion.
Organ or Tissue Perforation
Organ or tissue perforation is a severe complication where a hernia mesh erodes through surrounding tissue, creating a hole in an organ or the abdominal wall. This occurs when the mesh’s rigid edges or its pressure on tissue leads to erosion over time. Perforations can cause severe internal injuries, including infection, internal bleeding, and the spillage of intestinal contents into the abdominal cavity, potentially leading to peritonitis, a life-threatening internal infection.
Revision Surgery
Patients may require hernia mesh revision surgery due to complications arising from the initial mesh implantation, such as mesh failure, infection, chronic pain, or mesh migration. Revision surgery involves removing or replacing the problematic mesh to address these issues.
Revision surgery aims to alleviate the complications experienced by the patient, restore the integrity of the hernia repair, and improve the patient’s quality of life. However, undergoing revision surgery can be more challenging than the initial procedure due to scar tissue, increased risk of infection, and the complexity of removing or replacing the surgical mesh implant, leading to a longer recovery period and additional emotional and financial strain for the patient.
Seroma
Seroma is the accumulation of serous fluid, which resembles blood plasma, in a tissue cavity, often developing after surgical procedures, including hernia mesh implantation. This fluid buildup occurs when the body’s natural response to surgery leads to the production of excess fluid that collects in the space created by the removal or displacement of tissue. In some cases, large or persistent seromas may require medical intervention, such as drainage, to alleviate symptoms.

Liable Parties in Atlanta and Sandy Springs Hernia Mesh Lawsuits
In hernia mesh lawsuits, several manufacturers are currently facing multidistrict litigation (MDL) due to complications associated with their products.
Atrium is dealing with 2,770 ongoing cases related to its C-Qur mesh products, indicating a significant number of patients have experienced adverse effects.
Bard Davol is another major manufacturer under scrutiny, with a staggering 21,169 Bard hernia mesh lawsuit cases pending regarding its polypropylene hernia mesh products, highlighting widespread concerns over their safety and efficacy.
Ethicon also faces hernia mesh MDLs, with 194 hernia mesh cases specifically targeting the Ethicon Flexible Composite Physiomesh, pointing to issues unique to this product.
Bard Hernia Mesh Lawsuit
Bard hernia mesh lawsuits have been filed by numerous plaintiffs who allege that Bard hernia mesh products caused serious complications, including pain, infections, and failure. These cases for allegedly defective hernia mesh products have been consolidated into a Bard hernia mesh MDL (Multi-District Litigation) to streamline the hernia mesh trial process and address common issues in a coordinated manner. As part of the Bard hernia mesh MDL, a third bellwether trial is underway, serving as a hernia mesh test trial to help both plaintiffs and defendants gauge how juries might respond to evidence and arguments presented in the broader Bard hernia mesh lawsuit. The outcome of the third bellwether trial could significantly impact the resolution of the remaining cases within the Bard hernia mesh MDL.
Covidien Hernia Mesh Lawsuit
Covidien hernia mesh lawsuits have been filed by individuals who experienced severe complications, such as mesh erosion and infection, allegedly due to defects in Covidien’s hernia mesh products. These cases have been consolidated into a Covidien hernia mesh MDL (Multi-District Litigation) to streamline pretrial proceedings and address common issues. The Covidien hernia mesh cases within the Covidien hernia mesh MDL could significantly impact the outcomes for all plaintiffs involved as they seek to hold the manufacturer accountable for the alleged harm caused by its products.
Atrium Hernia Mesh Lawsuit
The Atrium hernia mesh lawsuit involves claims from plaintiffs who allege that Atrium’s hernia mesh products led to serious complications, including infections and failure. As the hernia mesh litigation progresses, a key hernia mesh trial has been conducted to determine the company’s liability. The results of this and other hernia mesh trials are critical in shaping the outcomes of the numerous cases filed against Atrium.

Do I Qualify for the Hernia Mesh Lawsuit in Georgia?
Determining eligibility for a hernia mesh lawsuit involves meeting specific criteria related to the complications and the timing of the original surgery. You may qualify if you:
- Were informed by a surgeon that additional surgery is necessary due to complications stemming from the hernia mesh.
- Have an additional hernia surgery scheduled to address complications related to the hernia mesh.
- Underwent the original hernia repair surgery with mesh on or after January 1, 2006.
- Experienced serious complications or injuries such as adhesions, hernia recurrence, intestinal blockage, mesh migration, organ perforation, or infection, which occurred more than 30 days after the original surgery date.
- Required hernia revision surgery or another surgical intervention to remedy complications associated with the mesh.
- Were advised that you need surgery to resolve complications caused by the hernia mesh but are unable to undergo the procedure due to other medical conditions.
These criteria aim to identify individuals who have suffered significant harm due to hernia mesh complications, ensuring that those most affected have a pathway to seek legal redress and compensation. If you meet the criteria to file a hernia mesh class action lawsuit, don’t hesitate to reach out to a Sandy Springs hernia mesh lawyer at Ashenden & Associates today.
Hernia Mesh Lawsuit Average Payout
The average hernia mesh payout can significantly vary based on a multitude of factors, such as the severity of the complications experienced by the patient, the extent of negligence involved on the part of the manufacturers or medical practitioners, and the overall damages incurred by the affected individual. The average hernia mesh lawsuit settlement can include compensation for medical bills, lost wages, pain and suffering, and other related costs.
Given the variability of each hernia mesh settlement, it’s crucial to have your case evaluated by knowledgeable hernia mesh lawyers who understand the intricacies of new hernia mesh lawsuits. The experienced hernia mesh lawyers at Ashenden & Associates are equipped to provide a thorough review of your case, taking into account all relevant details to offer an estimated hernia mesh settlement you might expect upon the successful resolution of your lawsuit. Our legal expertise ensures that every aspect of your case is meticulously considered to secure the hernia mesh lawsuit settlement you rightfully deserve.
Hernia Mesh Lawsuit Damages
Hernia mesh victims can recover a wide variety of damages following their lawsuit, including economic and non-economic damages.

Economic Damages in Hernia Mesh Settlements
Economic damages in hernia mesh lawsuits refer to the tangible, quantifiable financial losses that plaintiffs incur as a result of complications from their defective hernia mesh product. These damages are intended to compensate for out-of-pocket expenses and financial setbacks, including:
- Medical Bills: Costs associated with additional surgeries, hospital stays, medications, and any other medical treatments needed to address complications from the hernia mesh.
- Lost Wages: Compensation for income lost due to time off work for recovery, medical appointments, and any resulting inability to perform work-related tasks.
- Future Medical Care: Estimated costs for ongoing medical treatments, therapies, or surgeries required in the future due to long-term complications from the hernia mesh.
- Lost Earning Capacity: Compensation for the plaintiff’s diminished ability to earn income in the future as a direct result of the complications experienced from the hernia mesh.
These economic damages included in hernia mesh settlements ensure that individuals affected by hernia mesh complications are made whole for the financial burdens they have faced due to the faulty medical device.
Non-Economic Damages in Hernia Mesh Lawsuit Settlements
Non-economic damages in hernia mesh lawsuits address the intangible, non-financial losses suffered by individuals due to severe injuries from hernia mesh surgeries. These damages are aimed at compensating for the personal and emotional impact of the complications, including:
- Pain and Suffering: Compensation for the physical pain and discomfort experienced as a result of hernia mesh complications.
- Emotional Distress: Recognition of the mental anguish, anxiety, depression, and other psychological effects stemming from the medical issues and their impact on daily life.
- Loss of Consortium: Compensation for the negative effects on the relationship between the plaintiff and their spouse or partner due to the complications from the hernia mesh.
- Loss of Enjoyment of Life: Recognition of the diminished ability to enjoy hobbies, recreational activities, and other aspects of life that were affected by the complications.
Non-economic damages are vital in acknowledging and compensating for the profound personal impact of hernia mesh complications beyond the financial costs incurred.
Hernia Mesh Lawsuit Settlements and Verdicts
Past hernia mesh lawsuit settlements and verdicts can provide insight into the potential outcomes of such cases, with some notable examples include:
In August 2022, a state jury in Rhode Island ruled against Becton Dickinson, a subsidiary of C.R. Bard, resulting in a $4.8 million award at the conclusion of a Bard hernia mesh lawsuit.
Antonio Milanesi received a $255,000 award in April 2022 from a jury verdict against C.R. Bard during the second bellwether trial.
Atrium reached a confidential agreement in July 2021, with some reports suggesting the Atrium hernia mesh MDL settlement amount was around $66 million.
Ethicon also entered into a confidential settlement in September 2021, the details of which remain undisclosed.
In June 2011, Bard Davol concluded a settlement for 2,600 Kugel hernia patch lawsuits, amounting to $184 million.
These cases highlight the varying outcomes of hernia mesh litigation, reflecting the individual circumstances of each case and the legal arguments presented.
Hernia Mesh Lawsuit Update 2024
As of August 2024, the hernia mesh MDLS (multidistrict litigation) have seen a significant escalation, with over 21,000 new hernia mesh lawsuits added and filed, illustrating the widespread impact of hernia mesh complications and signaling ongoing concerns about the safety and efficacy of these medical devices.
In a notable development, the next hernia mesh trial in the Bryan case, initially scheduled for April 2024, has been canceled. The presiding judge has instead urged all involved parties to pursue a hernia mesh settlement. This case centers on the plaintiff, who has endured chronic pain since the implantation of a 3DMax mesh in 2012, leading to a partial explant in 2017. The plaintiff contends that the defendant continued to market the surgical mesh despite being aware of its potential risks and failing to provide sufficient warnings to consumers and medical professionals.
Furthermore, February 2024 marks the commencement of the fourth bellwether trial, a critical juncture that could influence the direction and outcomes of future hernia mesh litigation.
Is the Hernia Mesh Lawsuit Still Going On?
Yes, Bard hernia mesh lawsuits, Covidien hernia mesh lawsuits, Atrium hernia mesh lawsuits, and Ethicon hernia mesh lawsuits are still ongoing. New claims are being filed as more individuals come forward with complications related to hernia mesh products.
How to File a Hernia Mesh Lawsuit in Sandy Springs or Atlanta
To join the ongoing Bard hernia mesh MDL, it’s essential to first confirm your eligibility for legal action, which can be determined through a complimentary case evaluation with a Sandy Springs personal injury attorney at Ashenden & Associates. Building a compelling case is the next step, requiring the collection of substantial evidence such as medical records and personal testimony to substantiate your claims. It’s crucial to file your defective hernia mesh lawsuit in adherence to the statutes of limitations and any specific state-imposed deadlines to ensure your case is considered valid. Following the filing, the negotiation process for a hernia mesh settlement with the defendant(s) will take place, aiming for a fair resolution. Should these negotiations not result in an agreeable hernia mesh settlement, the case may then proceed to a hernia mesh test trial, where you’ll have the opportunity to present your case before a judge or jury for a final verdict.
Statute of Limitations for Hernia Mesh Lawsuit in Georgia
The statute of limitations for filing hernia mesh lawsuits varies by state, with each jurisdiction setting its own time limits for initiating legal action. In Georgia, for instance, the statute of limitations commences either on the date the harm from the hernia mesh occurs or on the date the harm should have reasonably been discovered by the plaintiff. It’s important to note that in cases of death resulting from hernia mesh complications, the statute of limitations extends to one year from the date of death. Additionally, Georgia law includes a 10-year statute of repose, which sets an ultimate deadline for filing claims, albeit with certain exceptions that may apply.
Given these complexities, it’s crucial to consult with an experienced Sandy Springs hernia mesh lawyer at Ashenden & Associates. Our legal expertise can ensure that your hernia mesh lawsuit is filed within the appropriate legal timeframe, safeguarding your right to seek compensation for your injuries.

Why You Need a Sandy Springs Hernia Mesh Lawyer
Navigating a hernia mesh lawsuit requires specialized legal expertise, making it essential to have experienced Sandy Springs hernia mesh lawyers by your side. Here’s why legal representation is invaluable:
- Legal Expertise: With years of experience in personal injury and product liability law, our attorneys possess a deep understanding of the complexities involved in hernia mesh cases.
- Personalized Attention: We provide personalized attention to each hernia mesh lawsuit, ensuring that your unique circumstances are effectively represented.
- Resource Accessibility: Access to a network of medical experts and resources is crucial for building strong hernia mesh claims, and Ashenden & Associates has the necessary connections to support your claim.
- Negotiation Skills: Our skilled negotiators can effectively deal with large medical device manufacturers and insurance companies, striving to secure the best possible outcome for you.
- Trial Readiness: If your case goes to a hernia mesh trial, you’ll benefit from having seasoned litigators who are prepared to advocate zealously on your behalf in court.
- No Upfront Costs: Our Sandy Springs hernia mesh lawyers work on a contingency fee basis, meaning you won’t pay legal fees unless you receive a settlement or verdict from the hernia mesh trial in your favor.
Having Sandy Springs hernia mesh lawyers from Ashenden & Associates on your side can significantly enhance your chances of achieving a favorable resolution, providing the support and expertise necessary to navigate hernia mesh litigation successfully.
Sandy Springs Hernia Mesh Lawyers
If you or a loved one has suffered hernia mesh complications, it’s crucial to take immediate action. The skilled Sandy Springs hernia mesh lawyer group at Ashenden & Associates can provide the support and expertise you need to pursue a hernia mesh claim in Atlanta or Sandy Springs. With a strong track record in handling hernia mesh lawsuits and other complex product liability cases, our Sandy Springs surgical mesh injury attorneys are committed to fighting for the justice and compensation you deserve.
Contact the Sandy Springs defective medical device lawyers at Ashenden & Associates at 770-394-8909 and take the first step towards holding negligent hernia mesh manufacturers accountable for their actions.
